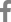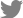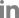CASE
A 65-year-old cancer survivor presented with widely fluctuating blood pressure (BP) over the past 2 years. At the age of 58, he was diagnosed with laryngeal cancer and completed chemoradiation. Two years later, the cancer recurred and was surgically removed. At the age of 63, he had surgery for degenerative lumbar spine disease. During the hospitalization, frequent episodes of high BP were noticed. During a typical episode, systolic BP (SBP) went above 180 mmHg, and he had flushing, headaches, choking sensation in the neck, and urge to urinate or defecate. He could not identify what triggered high BP episodes. He tried clonidine patch, oral clonidine, and lisinopril. None of the medications helped. He found that inhaled medical marijuana during episodes was sometimes helpful. SBP occasionally dropped down to 80 mmHg with lightheadedness while he was resting. Review of system was unremarkable except for transient postural dizziness and paresthesia in distal parts of limbs. Various diagnostic tests including serum metanephrines, cortisol, renin, aldosterone, thyroid-stimulating hormones, 24-hour urinary catecholamines and metabolites, echocardiogram, polysomnogram, and renal artery Doppler were unremarkable. During physical examination, BP was 198/112 mmHg in a seated position with heart rate (HR) of 71 bpm. His voice was hoarse due to the cancer treatment. He became flushed and restless due to high BP at the beginning of the clinic session. He excused himself to inhale marijuana outside and came back. Neurological examination was unremarkable with no evidence of parkinsonism or sensory small-fiber neuropathy. Autonomic function test showed minimal changes in HR during Valsalva maneuver (VM), orthostatic hypotension on the tilt-table test, and excessive hypertensive response to cold stimulation (Fig.).
Question 1. What are the differential diagnoses of labile hypertension?
BP is a hemodynamic measurement that fluctuates constantly in response to various internal and external stimuli. There is no widely accepted consensus on how to define labile hypertension (HTN) and quantify its fluctuation. Labile HTN is characterized by recurrent and sudden hypertensive episodes intermixed with low or normal BP. Labile HTN is thought to be associated with uncontrolled sympathetic adrenergic activities. Its symptoms include headaches, flushing, palpitations, urge to urinate, and encephalopathy. To demonstrate labile HTN, a 24-hour ambulatory BP monitoring may be required. Various diseases can cause labile HTN as shown in Table 1.
Our patient already completed extensive cardiovascular, endocrine, and sleep tests. Intermixed hypotension and absence of consistent emotional triggers excluded psychological conditions. He was not on a drug that may cause HTN. He denies chronic pain, snoring, and excessive daytime sleepiness. Pseudopheochromocytoma is a diagnosis of exclusion and more common in younger females. The history of neck radiation and surgery for laryngeal cancer years before the presentation and the extensive negative tests for non-neurological conditions warranted further evaluation for a primary autonomic disorder.
Question 2. What autonomic disorders can cause labile BP?
The main mechanism of labile BP in primary autonomic disorders is impairment in the arterial baroreflex [1]. Arterial baroreflex is an essential feedback mechanism that controls BP. The baroreceptors in the carotid sinus, the right cardiac atrium or the great vessels of the thorax cavity sense changes in BP. Higher arterial pressure stretches the blood vessel wall and activates the stretch-sensitive mechanoreceptors. The receptors send out signals to the brainstems via the glossopharyngeal and the vagus nerves (the afferent limb of the baroreflex). The brainstem processes the incoming signals with modification from the higher-order brain structures, then transmits outgoing signals to the heart and the blood vessel via the cardiovagal and sympathetic adrenergic pathways (the efferent limb). The efferent signals modulate HR and BP accordingly. Interruption in either limb or brainstem lesions can cause wide fluctuation in BP. Autonomic disorders due to efferent baroreflex dysfunction, traditionally called autonomic failure, result in disrupted BP control leading to neurogenic orthostatic hypotension and postprandial hypotension. A significant amount of blood shifts into the lower body (upright position), the intestine (eating), the skin (high temperature), or the muscle (exercise) decreases systemic BP because compensatory vasoconstriction via the efferent pathways is disabled in autonomic failure. About half of patients with neurogenic orthostatic hypotension have supine hypertension making BP fluctuate more widely. Autonomic neuropathies in various conditions including diabetes mellitus, vitamin B12 deficiency, Sjögren’s disease, and amyloidosis can cause autonomic failure. Alpha-synucleinopathies such as Parkinson’s disease may cause autonomic failure in older population [2]. Afferent baroreflex failure (ABF) has clinical features of episodic hypertension (hypertensive crises), hypotensive episodes, and orthostatic hypotension. Labile HTN or recurrent hypertensive crisis is the most common presentation. Hypertensive crises may occur spontaneously or in response to various stimulations. Mental stress from emotional distress or mental tasks is the most common trigger. Physical activities and cold environment can be triggers as well. Some patients with ABF also experience intermittent hypotensive episodes. Mechanisms of hypotension in ABF are unclear. Hypovolemia from pressure-induced diuresis, postprandial hypotension, orthostatic hypotension, and BP-lowering agents may contribute to hypotensive episodes. The most common cause of ABF is radiation to treat head and neck cancers [3]. Other causes are neck surgeries, familial dysautonomia, carotid endarterectomy, and brainstem stroke [3-6].
In our case, the key factor in the history was neck radiation and surgery 3-5 years before labile HTN. ABF causing labile HTN may occur years after neck radiation, whereas it happens much sooner following surgeries [3]. It is not clearly explained why ABF is delayed and occurs to only a small proportion of patients after neck radiation. Physical examination revealed no evidence of small-fiber neuropathy or Parkinsonism. He denied prodromal symptoms of Parkinson’s disease such as dream enactment, constipation, and hyposmia. Therefore, ABF was on the top of differential diagnoses, and evaluation of arterial baroreflex was needed to support the diagnosis. Other neurological conditions such as migraine and hyperadrenergic postural orthostatic tachycardia were likely to have normal baroreflex function during a test described below.
Question 3. What neurophysiological test can be used to assess baroreflex?
The modified Oxford maneuver is considered a gold standard test for evaluation of arterial baroreflex. It is a pharmacological method with sodium nitroprusside injection followed by phenylephrine under continuous BP and HR monitoring [7]. But it is unavailable in clinical settings. VM is a more feasible tool to assess baroreflex. Under beat-to-beat BP and HR monitoring, a patient is asked to blow through a mouthpiece at 40 mmHg (or 54 cmH2O) for 15 seconds. VM induces four distinct BP phases. At the beginning, intrathoracic pressure increases, which shifts blood from the thoracic cavity into the peripheral circulation. It results in transient increase BP and reciprocal decrease in HR (phase 1). Due to the high intrathoracic pressure, venous return and cardiac output decrease with resulting drop in arterial BP (early phase 2). The lowered BP activates the baroreflex leading to adrenergic vasoconstriction (late phase 2). HR increases initially through baroreflex-mediated cardiovagal withdrawal, later by cardiac sympathetic activation during phase 2. When the blowing stops, intrathoracic pressure is normalized resulting in a transient drop in BP due a passive mechanical effect (phase 3). A large amount of venous blood previously shifted to the periphery returns to the heart, which pumps out the blood at the accelerated rate. This creates BP overshooting that induces reciprocal decrease in HR by baroreflex cardiovagal activation (phase 4) (Fig. A). In ABF, lack of afferent signals about BP changes during phase 2 and phase 4 renders HR unable to respond.
During VM in our case, there was a persistent drop in BP without recovery during phase 2 and no overshooting. While BP dropped during phase 2 and gradually increased to the baseline during phase 4, the HR curve stayed unchanged (Fig. B). The abnormal hemodynamic response indicated baroreflex dysfunction. But these findings did not reliably differentiate ABF from (efferent) autonomic failure.
Question 4. What tests can tell between afferent and efferent baroreflex dysfunction?
Tests for pressor response can be helpful for the differentiation. Mental tasks, handgrip or hand immersion into cold water increase BP in healthy subjects (pressor response), and normal baroreflex prevents excessive BP surge. In ABF, once pressor response is induced, lacking afferent signals about rising BP leaves baroreflex incapacitated resulting in unconstrained increase in BP. In autonomic failure, efferent defects in sympathetic adrenergic pathways reduce pressor response. For a diagnosis of ABF, hand immersion into cold water, also known as cold pressor test, is preferred because other pressor tests such as mental tasks and handgrip involve cortical or central activation. Exaggerated BP response to cold pressor stimulation can be seen in patients with essential hypertension as well. But hemodynamic response during VM would be normal in essential hypertension.
In our case, the patient had a tilt-table test during which a cold pressor test was performed. In the resting and supine state before tilting up, there was spontaneous and wide fluctuation in BP. Upon tilting up, BP dropped meeting criteria for orthostatic hypotension (Fig. C). There was a brief increase in BP while the patient was talking. The cold pressor test showed a boost in SBP by at least 70 mmHg (Fig. D). The exaggerated BP response to cold pressor as well as absence HR changes during VM was consistent with ABF. Spontaneous large oscillations in BP during a resting state and orthostatic hypotension can be seen in ABF as well.
Question 5. How to manage blood pressure in ABF?
Labile HTN in ABF is extremely difficult to control. Patients and providers need to understand that it is impossible to remove BP fluctuation in most cases. Therefore, the BP management should be focused on reduction of hypertension crisis episodes, not on normalization of BP. Long-acting sympatholytics such as clonidine patch, guanfacine and methyldopa are preferred. Immediate release clonidine can be used for breakthrough HTN episodes. Its frequent use may cause rebound HTN. When emotional stress is an obvious trigger of hypertensive crises, benzodiazepine or cognitive behavior therapy can be considered. Medical marijuana can be an option depending on local laws. For hypotensive episodes, physical activities such as handgrip, oral water bolus, abdominal binder, or short-acting vasoconstrictor (midodrine) can be tried as a rescue therapy although they have not been studied systemically. Because pressure-induced natriuresis from hypertension results in hypovolemia and worsens hypotension, adequate hydration is recommended.
The patient started guanfacine 0.1 mg daily. Hypertensive episodes with symptoms occurred less frequently, although it was hard to tell without ambulatory BP monitoring if asymptomatic HTN episodes also decreased.
KEY POINTS
1. Variable medical conditions can cause labile hypertension. Among primary autonomic disorders, afferent baroreflex failure and neurogenic orthostatic hypotension with supine hypertension can be considered.
2. Valsalva maneuver test can assess baroreflex. But it does not differentiate afferent failure from efferent failure. Cold pressor test can be helpful for differentiation.
3. Labile hypertension due to afferent baroreflex failure is extremely hard to control. The primary goal of blood pressure management is to reduce or prevent dangerous blood pressure spikes.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print